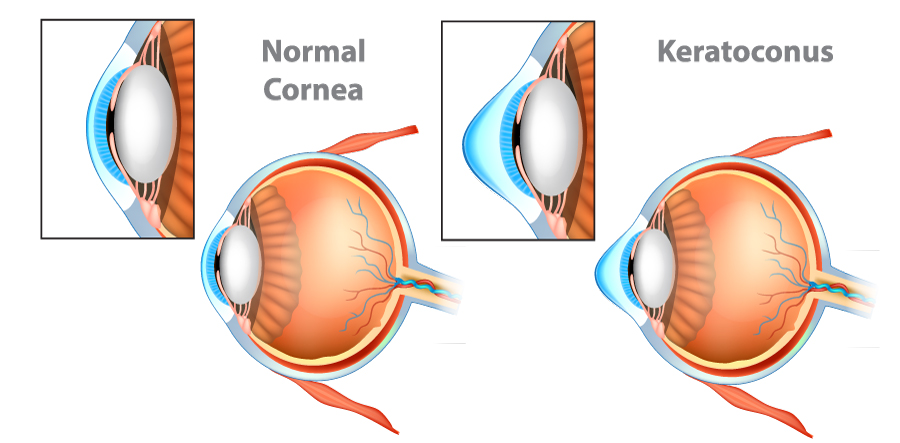
Collagen Cross-Linking is an FDA treatment for corneal disorders including keratoconus and corneal ectasia following refractive surgery. In a healthy eye, the cornea is a clear window to the eye that is shaped with a gradual and symmetric curve, like a dome. This set of disorders all can cause thinning and steepening of the cornea. Specifically, keratoconus is a degenerative and progressive disease that leads to distorted vision as the cornea becomes more misshapen and cone-shaped in time. These changes can be subtle, but individuals may notice a progressive worsening of vision, distortion to their vision, double vision, or notice an increase in light sensitivity. Cross-linking is a new therapy in the US aimed at halting the progression of keratoconus.
Historically, treatment options for keratoconus were limited. Hard contact lenses, Intacs (half-moon shaped implants that flatten the shape of the cornea; see image below), and corneal transplantation were the most common ways to treat keratoconus.
In April of 2016, the FDA approved Collagen Cross-Linking (KXL) for treatment of progressive keratoconus. KXL Treatment, although more recently approved in the US, this procedure has been performed in Europe since 2003, and by providers in Canada since 2008. The goal of KXL is to halt the progression of keratoconus. During the treatment, the cornea’s epithelial cell layer is gently removed. A medicine comprised of Riboflavin, also known as Vitamin B2, is then applied to the eye. A special UV light is directed onto the eye. This treatment strengthens the collagen bonds of the cornea, increasing the rigidity and stability of the cornea.
As with any surgery, there are some risks. The risks of Collagen Cross-Linking include corneal haze, corneal ulcers and epithelial defects. Patients are monitored closely after KXL treatments to reduce the risk of these side effects.


